


















table relative strength of selected acids and their conjugate bases acid strongest acid hsbf6 hi h2so4 hbr hcl c6h5so3h (ch3)2oh (ch3)2c oh weakest acid -12 -10 Question: Draw The Conjugate Base For The Acid (CH3)2C=OH+. Remember To Include Charges And Non-bonding Electrons Where Necessary. This problem has been solved! See the answer. Show transcribed image text. Expert Answer . Previous question Next question Transcribed Image Text from this Question. Draw the conjugate base for the acid (CH3)2C=OH+. Remember to include charges and non-bonding ... 1) the conjugate acid of the base CH3CH2OH. you just need to add an H+. CH 3 CH 2 OH 2 + 2) NH3 is the classic example of a lewis base. lewis acids accept electron pairs , NH3 has an electron pair to give. PBr3 has the same molecular geometry and lewis structure as NH3 so the same reason above. it will behave as a lewis base. i would says AlCl3 What are the conjugate bases of the following acids?: (a) HCO3- (b) (CH3)2NH2+ Not sure how to solve this (I imagine its simple) I don't know the techinque. If they are acids, they will lose a proton when they react. So take H + off the formula. eg HSO 4-----> SO 4 2- + H + acid conj base. Log in or register to post comments; Similar Questions. Identifying bronsted acids/bases: Conjugate acids ... Acid Conjugate Base (CH3)2C=OH+. This problem has been solved! See the answer. orgo hw help. Show transcribed image text. Expert Answer 86% (14 ratings) Previous question Next question Transcribed Image Text from this Question. Draw the conjugate base for the following acid. Remember to include charges and non-bonding electrons where necessary. Acid Conjugate Base (CH3)2C=OH+ ... base, and label it. (ii) Indicate whether the equilibrium lies to the left or the right. (iii) Estimate K for each reaction if possible. (Hint: Use the Evan’s pKa Table)† H2O HCN H3O+ CN-CH 3O-NH CHOH NH2-HF CH3COO-F-CH 3COOH C H3-NH C 4 NH2-H3O+ Cl-H 2O HCl CH 3COOH HS-CH 3COO-CH3SH Blue = Base K= Red = Acid-1.3 x 101-3.2 x 102 ~40 1010 2 ... ACID: (CH3)2C = OH+ (C H 3) 2 C = O H + There is one pair of lone electrons on the oxygen. Acid and Conjugate Base The conjugate acid is obtained when the base gains one proton.
[index] [1335] [6120] [5576] [7001] [8104] [2668] [7636] [3897] [2186] [6587]
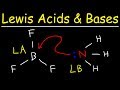
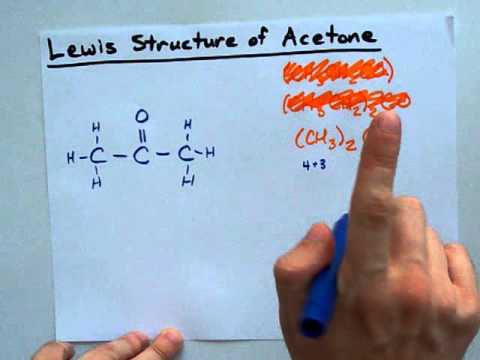
Trick to Find Conjugate Acid and Conjugate Base / Ionic Equilibrium Tricks Conjugate acids and bases are usually introduced in organic chemistry along with a review of resonance. Category Education; Show more Show less. Loading... Autoplay When autoplay is enabled, a ... A step-by-step explanation of how to draw the Acetone Lewis Structure. Acetone is a member of the Ketone family of organic compounds. It is the simplest ... Acetone is (CH3)2CO ... aka C3H6O ... here I show you how to draw its Lewis Structure.It's pretty straightforward...I'm not exactly sure why it's a challeng... I quickly take you through how to draw the Lewis Structure of CH3COOH (Acetic Acid). I also go over hybridization, shape, sigma, pi bonding and bond angles. A step-by-step explanation of how to draw the HNO3 Lewis Structure (Nitric Acid). The HNO3 Lewis structure is best thought of as the NO3 with an H attache... This organic chemistry video tutorial provides a basic introduction into lewis acids and bases. It explains how to predict the products of a lewis acid-base... Use Bronsted Lowry Acid/Base Theory to identify conjugate acid base pairs.More free chemistry help at www.chemistnate.com Donate here: http://www.aklectures.com/donate.phpWebsite video link: http://www.aklectures.com/lecture/arrhenius-bronsted-lowry-and-lewis-acids-and-basesFace... A step-by-step explanation of how to draw the CH3CH2OH (C2H5OH) Lewis Dot Structure.For the CH3CH2OH Lewis structure, calculate the total number of valence e...
Copyright © 2024 m.newtoy.site